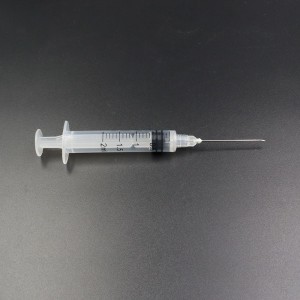
ʻO ka Syringe Hoʻolaha i ʻae ʻia e CE FDA ISO Auto-Disable Syringe

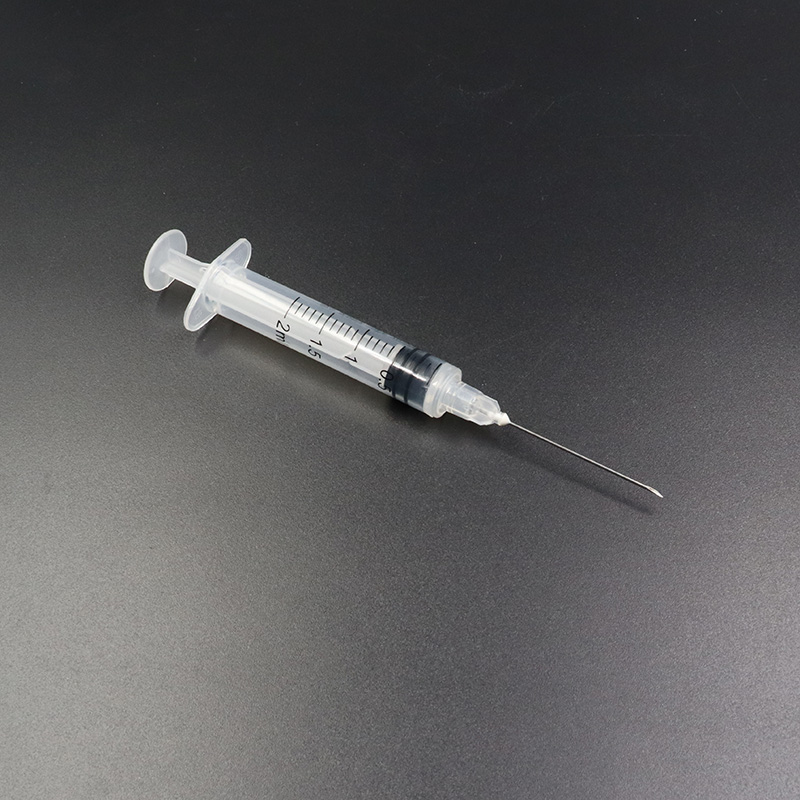
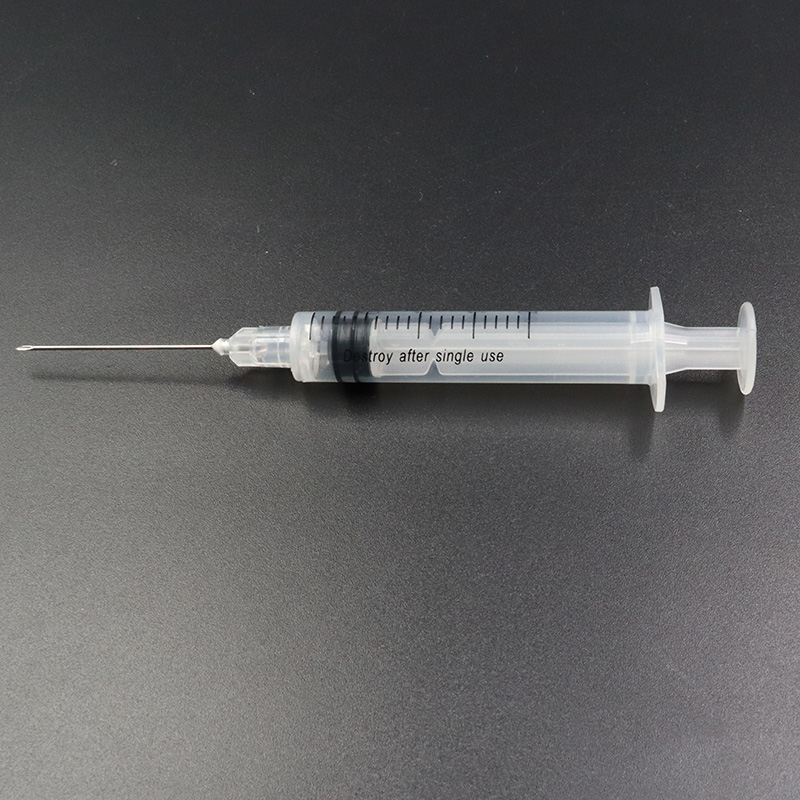
Ua hoʻolālā ʻia nā syringes hoʻopau ponoʻī e pale i ko lākou hoʻohana hou ʻana, a laila e hōʻemi ana i ka pilikia o ka hoʻolaha ʻana i nā maʻi a me nā maʻi. Hoʻohana pinepine ʻia kēia mau syringes i nā wahi mālama ola a he nui nā noi koʻikoʻi, e like me:
- Nā Hoʻolaha Palekana.
- Ke hāʻawi nei i nā lāʻau lapaʻau, nā lāʻau pale, a me nā inikini ʻē aʻe i nā maʻi.
- Hōʻiliʻili Koko.
- Hana Hana Lāʻau Lapaʻau.
ʻO ka Syringe Hoʻolaha i ʻae ʻia e CE FDA ISO Auto-Disable Syringe
Kikoʻī: 1ml, 2-3ml, 5ml, 10ml, 20ml, 30ml, 50ml;
Manaʻo kōkua: Paheʻe Luer;
Sterile: Ma ke kinoea EO, ʻAʻohe ʻawahia, ʻAʻohe Pyrogenic
Palapala hōʻoia: CE a me ISO13485
Nā pono huahana:
Hana lima hoʻokahi a me ka hoʻāla ʻana;
Noho nā manamana lima ma hope o ke kui i nā manawa a pau;
ʻAʻohe loli i ke ʻano hana inikini;
Hoʻokomo ka paheʻe Luer i nā nila hypodermic maʻamau āpau

CE
ISO13485
ʻAmelika Hui Pū ʻIa FDA 510K
EN ISO 13485: 2016/AC:2016 ʻŌnaehana hoʻokele maikaʻi o nā lako lapaʻau no nā koi hoʻoponopono
EN ISO 14971: 2012 Nā mea lapaʻau - Hoʻopili ʻana i ka hoʻokele pilikia i nā mea lapaʻau
ISO 11135:2014 Mea lapaʻau Sterilization o ka ethylene oxide Hōʻoia a me ka kaohi laulā
ISO 6009:2016 Nā nila inikini sterile hoʻolei ʻia E ʻike i ke code kala
ISO 7864:2016 Nā nila inikini sterile hoʻolei ʻia
ISO 9626:2016 Nā paipu nila kila kila no ka hana ʻana i nā mea lapaʻau

He alakaʻi ʻo SHANGHAI TEAMSTAND CORPORATION i nā huahana a me nā hoʻonā lapaʻau.
Me ka ʻoi aku o 10 mau makahiki o ka ʻike hoʻolako olakino, hāʻawi mākou i kahi koho huahana ākea, nā kumukūʻai hoʻokūkū, nā lawelawe OEM kūikawā, a me nā hoʻouna manawa kūpono. ʻO mākou ka mea hoʻolako o ke Keʻena Ola o ke Aupuni Australia (AGDH) a me ke Keʻena Ola o Kaleponi (CDPH). Ma Kina, kūlana mākou ma waena o nā mea hoʻolako kiʻekiʻe o Infusion, Injection, Vascular Access, Rehabilitation Equipment, Hemodialysis, Biopsy Needle a me Paracentesis.
Ma ka makahiki 2023, ua hoʻolako pono mākou i nā huahana i nā mea kūʻai aku ma nā ʻāina he 120+, me ʻAmelika Hui Pū ʻIa, EU, Hikina Waena, a me ʻAsia Hikina Hema. Hōʻike kā mākou hana i kēlā me kēia lā i ko mākou hoʻolaʻa a me ka pane ʻana i nā pono o nā mea kūʻai aku, e hoʻolilo ana iā mākou i hoa ʻoihana hilinaʻi a hoʻohui ʻia o ke koho.

Ua loaʻa iā mākou kahi inoa maikaʻi ma waena o kēia mau mea kūʻai aku a pau no ka lawelawe maikaʻi a me ke kumukūʻai hoʻokūkū.

A1: He 10 mau makahiki o ko mākou ʻike ma kēia kahua, he hui ʻoihana kā mākou hui a me ka laina hana ʻoihana.
A2. ʻO kā mākou huahana me ke kūlana kiʻekiʻe a me ke kumukūʻai hoʻokūkū.
ʻO A3.ʻO ka maʻamau he 10000pcs; makemake mākou e hana pū me ʻoe, mai hopohopo e pili ana i ka MOQ, e hoʻouna wale mai iā mākou i kāu mau mea āu e makemake ai e kauoha.
ʻAe, ua ʻae ʻia ka hoʻopilikino LOGO.
A5: ʻO ka maʻamau mālama mākou i ka hapa nui o nā huahana i loko o ka waihona, hiki iā mākou ke hoʻouna i nā laʻana i loko o 5-10 mau lā hana.
A6: Hoʻouna mākou ma FEDEX.UPS, DHL, EMS a i ʻole ke Kai.