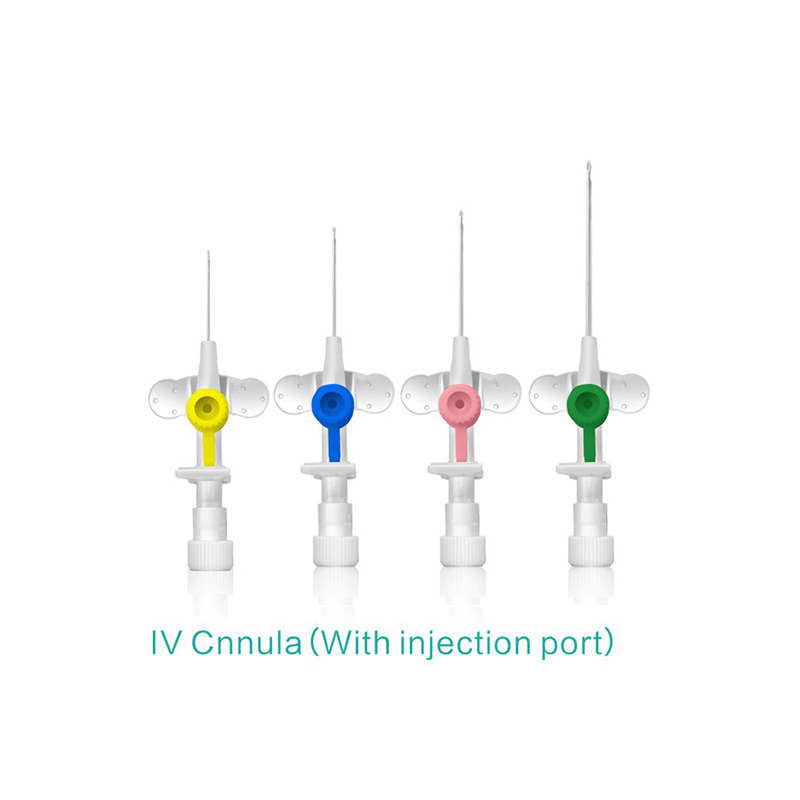
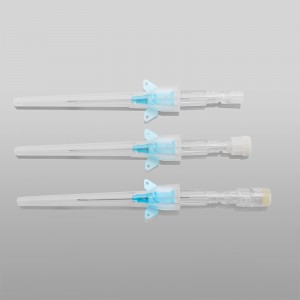
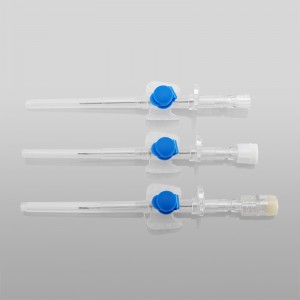
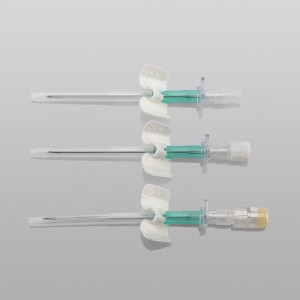
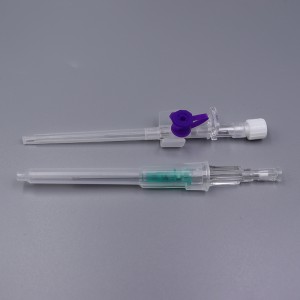
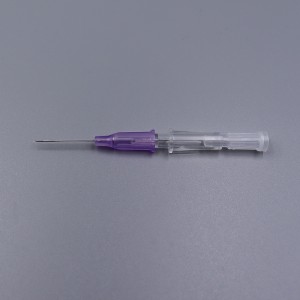
ʻO ka lako lapaʻau i hōʻoia ʻia e CE ISO FDA Disposable IV Cannula
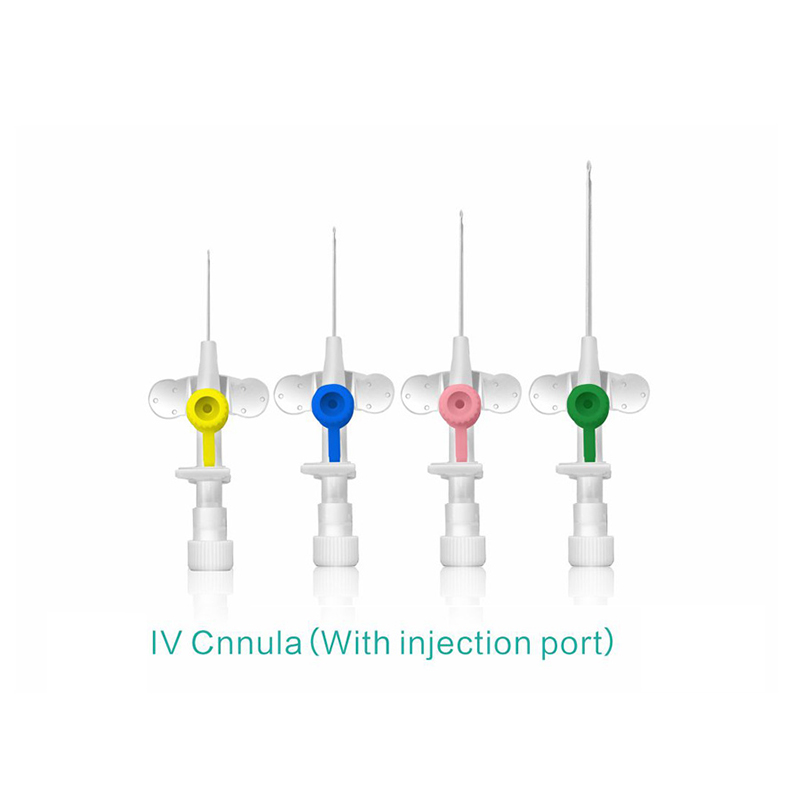
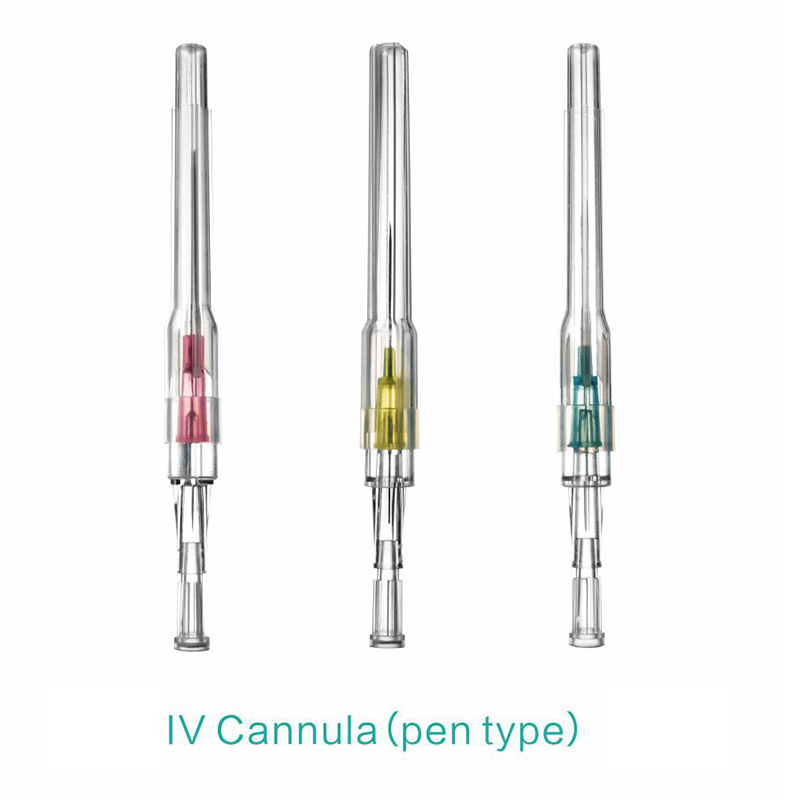
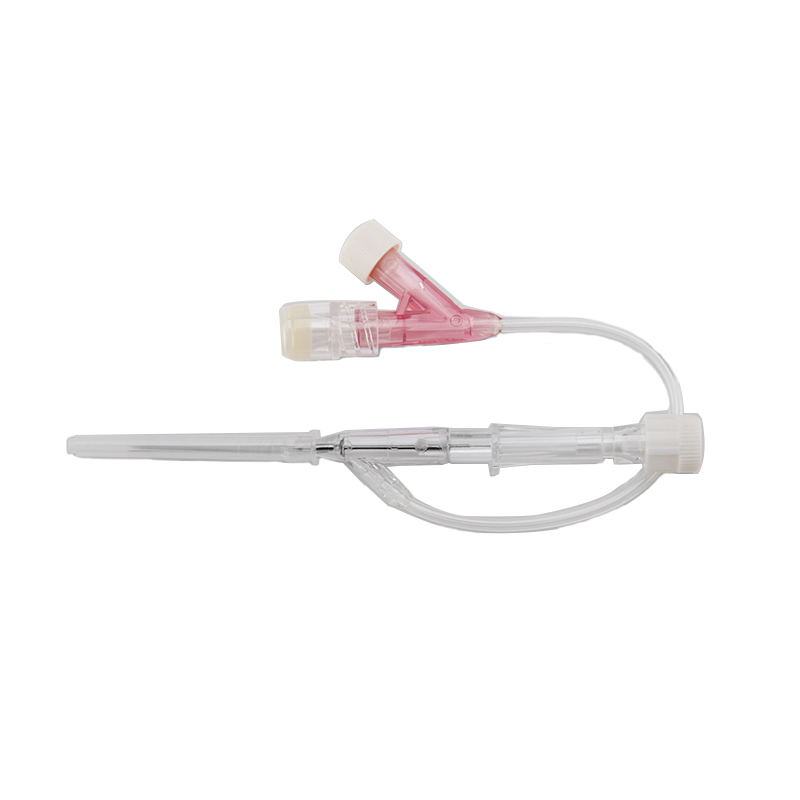
ʻO kahi intravenous (IV) cannula kahi mea lapaʻau i hoʻohana ʻia e hoʻokomo i nā wai, nā lāʻau lapaʻau, a me nā huahana koko i loko o ke aʻa o ka mea maʻi.
Nā kikoʻī
Nui: 14G, 16G, 18G, 20G, 22G, 24G a me 26G
Hiʻona
ʻO ke poʻi o ka pahu i hoʻololi ʻia i ke kala e maʻalahi ai ka ʻike ʻana i ka nui o ka catheter.
ʻO ke kikowaena catheter translucent a me ke keʻena flashback e ʻae i ka ʻike maʻalahi o ke koko.
ʻO ke catheter Teflon radio-opaque.
ʻO ka catheter PET a PU paha i hoʻopau pololei ʻia e hōʻoiaʻiʻo i ke kahe paʻa a hoʻopau i ka kink o ka catheters.
Hiki ke hoʻopili ʻia i ka syringe ma ka wehe ʻana i ke poʻi kānana e hōʻike i ka hopena taper lure.
Hoʻopau ka hoʻohana ʻana i ka kānana membrane hydrophobic i ke kahe koko.
ʻO ka pilina kokoke a laumania ma waena o ka piko catheter a me ka nila o loko e hiki ai ke palekana a laumania.
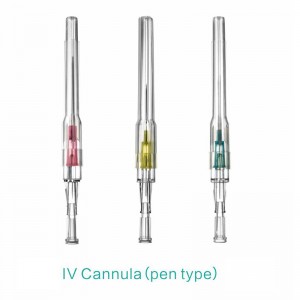
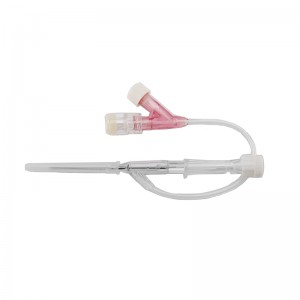
ʻAno peni, ʻano ʻēheu, ʻano pulelehua, ʻano Y, a pēlā aku.
He koho ke awa hoʻokomo.
CE
ISO13485
ʻAmelika Hui Pū ʻIa FDA 510K
EN ISO 13485: 2016/AC:2016 ʻŌnaehana hoʻokele maikaʻi o nā lako lapaʻau no nā koi hoʻoponopono
EN ISO 14971: 2012 Nā mea lapaʻau - Hoʻopili ʻana i ka hoʻokele pilikia i nā mea lapaʻau
ISO 11135:2014 Mea lapaʻau Sterilization o ka ethylene oxide Hōʻoia a me ka kaohi laulā
ISO 6009:2016 Nā nila inikini sterile hoʻolei ʻia E ʻike i ke code kala
ISO 7864:2016 Nā nila inikini sterile hoʻolei ʻia
ISO 9626:2016 Nā paipu nila kila kila no ka hana ʻana i nā mea lapaʻau

He alakaʻi ʻo SHANGHAI TEAMSTAND CORPORATION i nā huahana a me nā hoʻonā lapaʻau.
Me ka ʻoi aku o 10 mau makahiki o ka ʻike hoʻolako olakino, hāʻawi mākou i kahi koho huahana ākea, nā kumukūʻai hoʻokūkū, nā lawelawe OEM kūikawā, a me nā hoʻouna manawa kūpono. ʻO mākou ka mea hoʻolako o ke Keʻena Ola o ke Aupuni Australia (AGDH) a me ke Keʻena Ola o Kaleponi (CDPH). Ma Kina, kūlana mākou ma waena o nā mea hoʻolako kiʻekiʻe o Infusion, Injection, Vascular Access, Rehabilitation Equipment, Hemodialysis, Biopsy Needle a me Paracentesis.
Ma ka makahiki 2023, ua hoʻolako pono mākou i nā huahana i nā mea kūʻai aku ma nā ʻāina he 120+, me ʻAmelika Hui Pū ʻIa, EU, Hikina Waena, a me ʻAsia Hikina Hema. Hōʻike kā mākou hana i kēlā me kēia lā i ko mākou hoʻolaʻa a me ka pane ʻana i nā pono o nā mea kūʻai aku, e hoʻolilo ana iā mākou i hoa ʻoihana hilinaʻi a hoʻohui ʻia o ke koho.

Ua loaʻa iā mākou kahi inoa maikaʻi ma waena o kēia mau mea kūʻai aku a pau no ka lawelawe maikaʻi a me ke kumukūʻai hoʻokūkū.

A1: He 10 mau makahiki o ko mākou ʻike ma kēia kahua, he hui ʻoihana kā mākou hui a me ka laina hana ʻoihana.
A2. ʻO kā mākou huahana me ke kūlana kiʻekiʻe a me ke kumukūʻai hoʻokūkū.
ʻO A3.ʻO ka maʻamau he 10000pcs; makemake mākou e hana pū me ʻoe, mai hopohopo e pili ana i ka MOQ, e hoʻouna wale mai iā mākou i kāu mau mea āu e makemake ai e kauoha.
ʻAe, ua ʻae ʻia ka hoʻopilikino LOGO.
A5: ʻO ka maʻamau mālama mākou i ka hapa nui o nā huahana i loko o ka waihona, hiki iā mākou ke hoʻouna i nā laʻana i loko o 5-10 mau lā hana.
A6: Hoʻouna mākou ma FEDEX.UPS, DHL, EMS a i ʻole ke Kai.