Paipu Baluna Lapaʻau Lapaʻau Postpartum Hemostasis
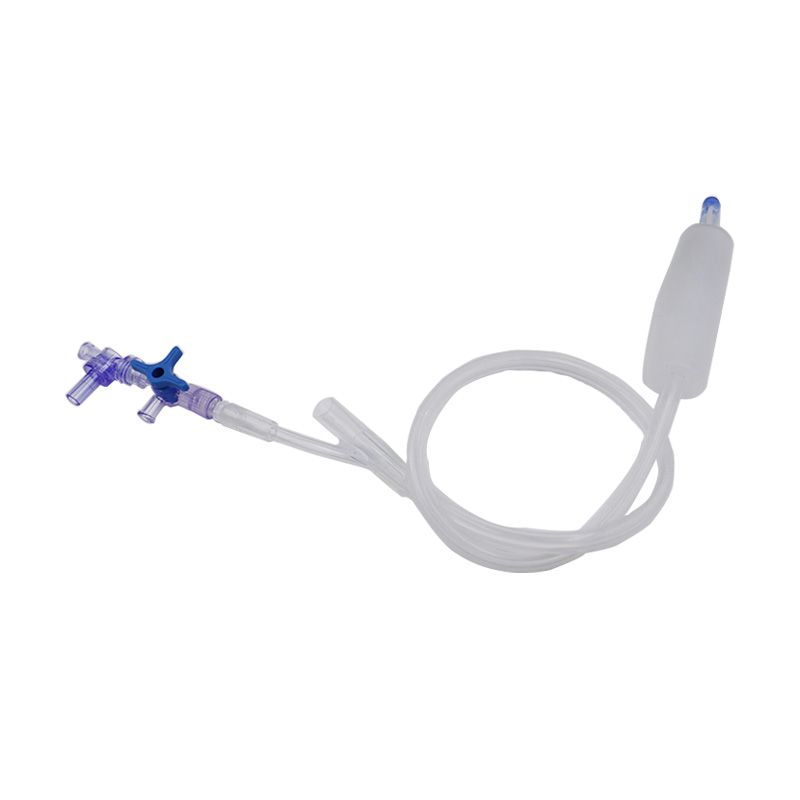

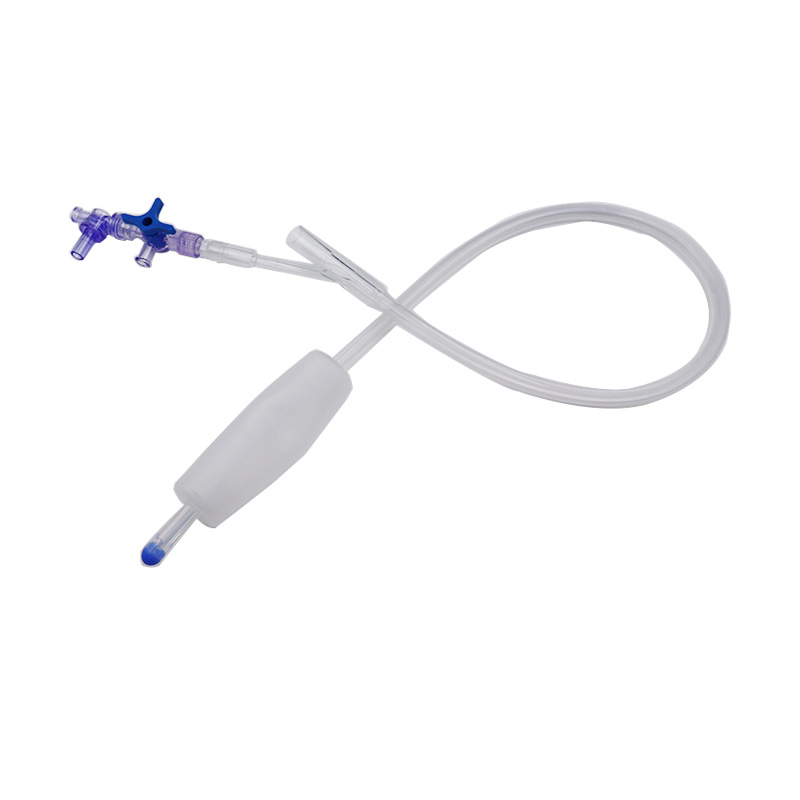
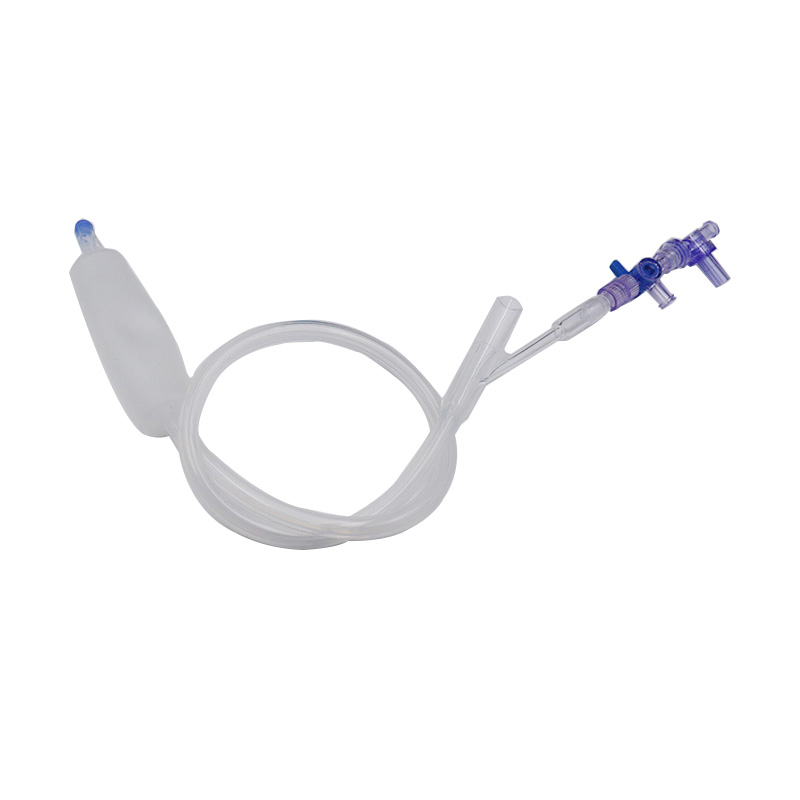
Hoʻohana ʻia ka Balloon Hemostasis Postpartum no ka hoʻomalu ʻana a i ʻole ka hōʻemi ʻana i ke koko o ka ʻōpū postpartum.
i ka wā e pono ai ka hoʻokele conservative. No ka mea, ʻoiai ua hoʻonui ʻia ka baluna me ka paʻakai sterilized, ʻo ka
Hoʻopili ka baluna postpartum i ke kaomi i nā paia o ka ʻōpū e kōkua i ka hemostasis.
Maʻalahi e hana a hoʻonohonoho;
ʻO ke ala palekana a maikaʻi hoʻi e hāpai i ka hemostasis;
Hiki ke hoʻohana ʻia no ka hānau ʻana a me ka cesarean;
Hoʻopau nā mea kino silicone i ka pilikia o ka maʻi maʻamau i ka latex;
ʻĀpana hoʻoemi ākea, hopena hoʻokahe maikaʻi;
He liʻiliʻi nā contraindications a me nā hopena ʻaoʻao.
MDR 2017/745
USA FDA 510K
EN ISO 13485: 2016/AC: 2016 ʻōnaehana hoʻokele maikaʻi o ka lāʻau lapaʻau no nā koi hoʻoponopono.
EN ISO 14971: 2012 Nā mea lapaʻau - Ka hoʻohana ʻana i ka hoʻokele pilikia i nā mea lapaʻau
ISO 11135: 2014 Mea lapaʻau Sterilization o ka ethylene oxide Hōʻoia a me ka mana maʻamau
ISO 6009: 2016 Nā nila hoʻoheheʻe sterile hoʻokuʻu e ʻike i ke code kala
ISO 7864: 2016 ʻO nā nila hoʻoheheʻe sterile hoʻopau
ISO 9626: 2016 ʻO nā paipu nila kila kila no ka hana ʻana i nā mea lapaʻau

He alakaʻi nui ʻo SHANGHAI TEAMSTAND CORPORATION i nā huahana lapaʻau a me nā hoʻonā.
Me ka ʻoi aku o 10 mau makahiki o ka ʻike hoʻolako olakino, hāʻawi mākou i kahi koho huahana ākea, nā kumu kūʻai hoʻokūkū, nā lawelawe OEM kūʻokoʻa, a me ka hāʻawi ʻana i ka manawa kūpono. ʻO mākou ka mea hoʻolako o ka Australian Government Department of Health (AGDH) a me California Department of Public Health (CDPH). Ma Kina, kū mākou i waena o nā mea hoʻolako kiʻekiʻe o Infusion, Injection, Vascular Access, Rehabilitation Equipment, Hemodialysis, Biopsy Needle a me nā huahana Paracentesis.
Ma ka 2023, ua hoʻopuka maikaʻi mākou i nā huahana i nā mea kūʻai aku ma 120+ mau ʻāina, me ka USA, EU, Middle East, a me Asia Hikina Hema. Hōʻike kā mākou mau hana i kēlā me kēia lā i ko mākou hoʻolaʻa ʻana a me ka pane ʻana i nā pono o ka mea kūʻai aku, e hoʻolilo iā mākou i hoa ʻoihana hilinaʻi a hoʻohui ʻia i koho.

Ua loaʻa iā mākou ka inoa maikaʻi ma waena o kēia mau mea kūʻai aku no ka lawelawe maikaʻi a me ke kumukūʻai hoʻokūkū.

A1: Loaʻa iā mākou he 10 mau makahiki ma kēia kahua, ʻO kā mākou hui he hui ʻoihana a me ka laina hana ʻoihana.
A2. ʻO kā mākou huahana me ke kūlana kiʻekiʻe a me ke kumukūʻai hoʻokūkū.
ʻO A3.ʻO ka maʻamau ka 10000pcs; makemake mākou e hui pū me ʻoe, ʻaʻohe hopohopo e pili ana i ka MOQ, e hoʻouna wale mai iā mākou i kāu mau mea āu e makemake ai e kauoha.
A4.Yes, ʻae ʻia ka hoʻopilikino LOGO.
A5: Ke mālama nei mākou i ka hapa nui o nā huahana i ka waihona, hiki iā mākou ke hoʻouna i nā laʻana ma 5-10workdays.
A6: Hoʻouna mākou e FEDEX.UPS, DHL, EMS a i ʻole Sea.












