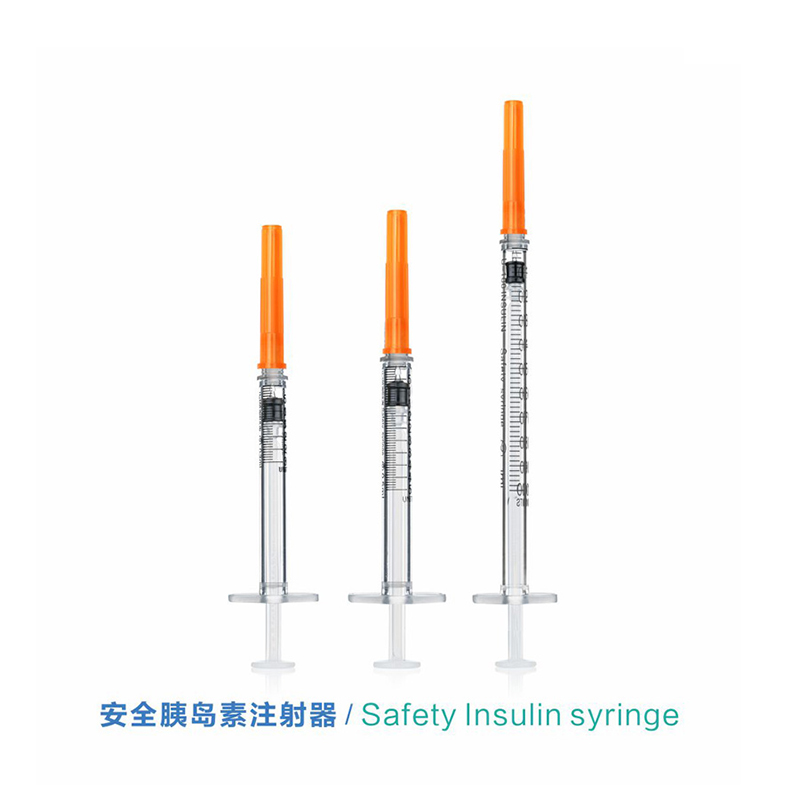
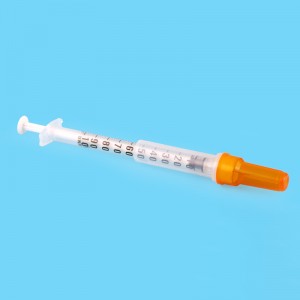
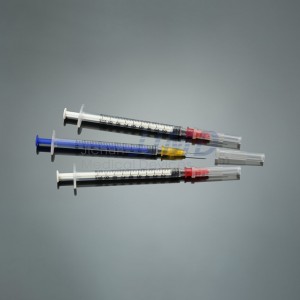
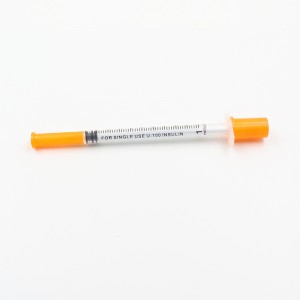
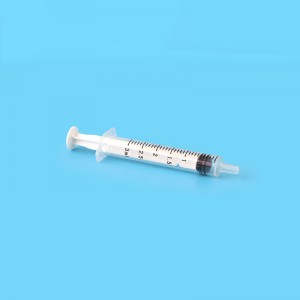
ʻO ka CE FDA Disposable Retractable Safety Insulin Syringe me ka Needle
Wikiō Huahana
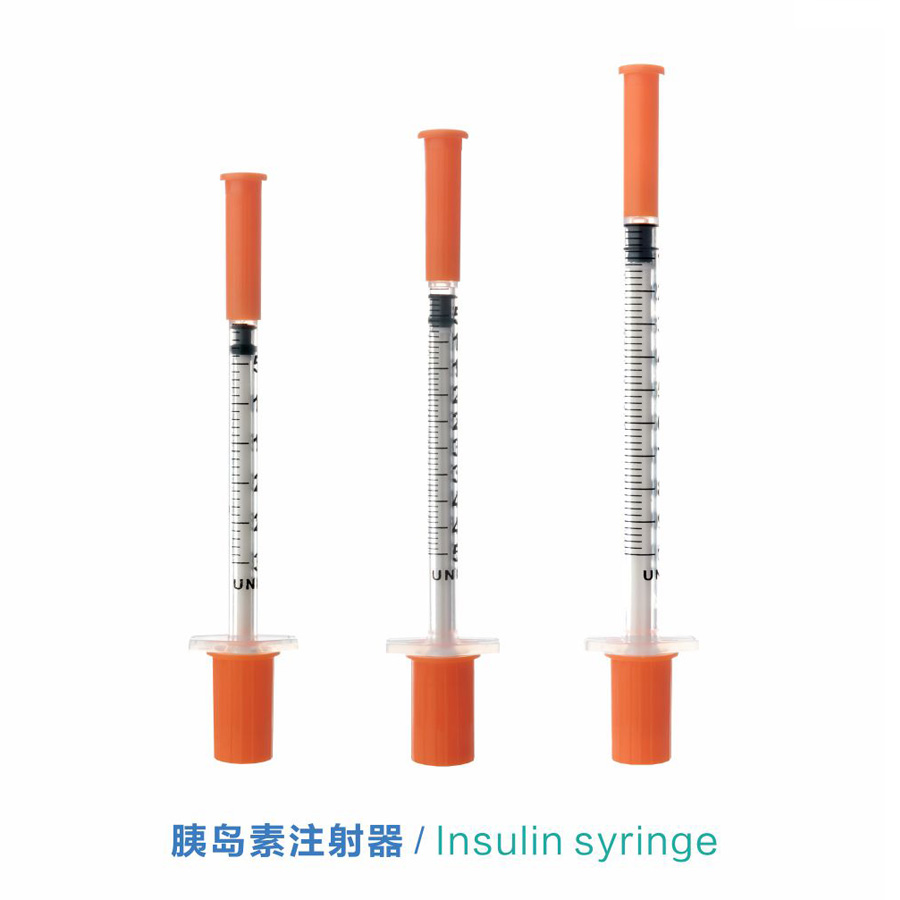
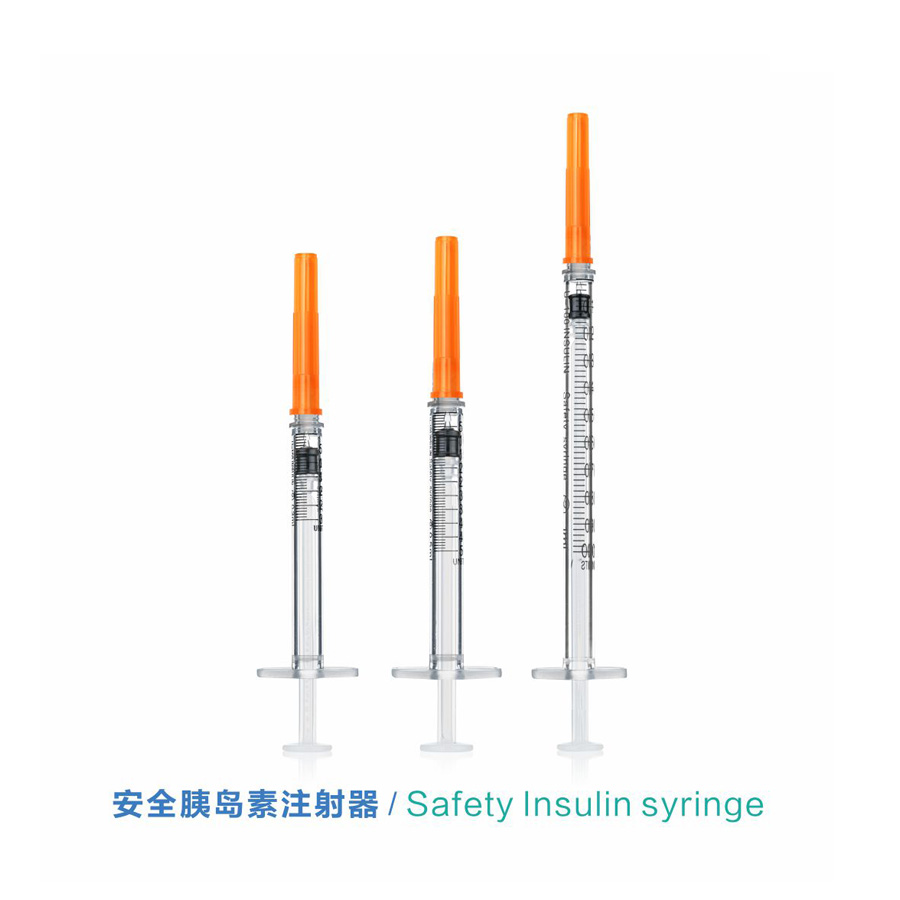
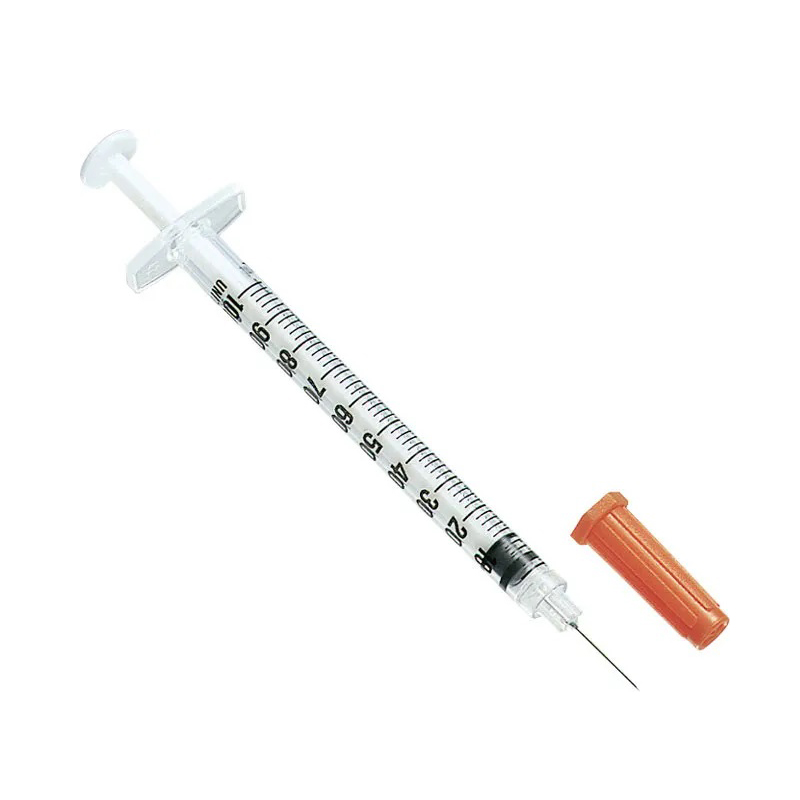
Wehewehena
1. Hana ʻia ka huahana i ka mea polymer lapaʻau.
2. Ua paʻa ka nila ma ka nozzle, ʻoi loa ka piko o ka nila, maopopo a pololei ka calibration, a hiki ke hoʻoholo pololei i ka dosage.
3.Mounted Needle, ʻAʻohe Make Make, ʻAʻohe ʻŌpala
4. ʻO ka barela moakaka lawa e hiki ai ke ana maʻalahi i ka nui i loko o ka syringe a me ka ʻike ʻana i ka pehu ea.
5. He maʻalahi ke heluhelu ʻana i ke ana helu puka ma luna o ka barela. Ua paʻi ʻia ke ana helu puka me ka ʻīnika mau loa.
6. Hoʻokomo maikaʻi loa ka plunger i loko o ka barela e ʻae ai i ka neʻe manuahi a laumania.
Hiʻona
ʻO ka syringe insulin hoʻolei ʻia nā nui like ʻole
HIʻONA
Kikoʻī: 0.3ml, 0.5ml a me 1ml (U-100 a i ʻole U-40)
Mea Hana: Hana ʻia me ka papa lapaʻau pp
Palapala hōʻoia: CE, palapala hōʻoia ISO13485
Pūʻolo: Pūʻolo blister
Nila: Nila paʻa
Unahi: Nā māka ʻāpana nui a maopopo
Maʻemaʻe: Ma ke kinoea EO
Ka nui: 0.3ml, 0.5ml a me 1ml.
Kuila: 29G, 30G a me 31G.
Nā kikoʻī
| Mea Hana | Cap&Barrel & Plunger: Papa lapaʻau PP |
| Nila: Kila kila | |
| Piston: Latex a i ʻole Latex Free | |
| Leo | 0.3ml,0.5ml,1ml |
| Noi | Lapaʻau |
| Hiʻona | Hoʻolei ʻia |
| Palapala hōʻoia | CE, ISO |
| nila | me ka nila paʻa a i ʻole ka nila i hoʻokaʻawale ʻia |
| Pūpū | ʻŌpū kikowaena |
| Kala plunger | Moakaka, keʻokeʻo, kala |
| Paila | Kiʻekiʻe ke aniani moakaka |
| Pūʻolo | Pūʻolo pākahi: Hoʻopili ʻia ka Blister/PE |
| Pūʻolo lua: Pahu | |
| Pūʻolo waho: Carton | |
| Maʻemaʻe | sterile e ke kinoea EO, ʻaʻohe ʻawahia, ʻaʻohe pyrogen |
CE
ISO13485
ʻAmelika Hui Pū ʻIa FDA 510K
EN ISO 13485: 2016/AC:2016 ʻŌnaehana hoʻokele maikaʻi o nā lako lapaʻau no nā koi hoʻoponopono
EN ISO 14971: 2012 Nā mea lapaʻau - Hoʻopili ʻana i ka hoʻokele pilikia i nā mea lapaʻau
ISO 11135:2014 Mea lapaʻau Sterilization o ka ethylene oxide Hōʻoia a me ka kaohi laulā
ISO 6009:2016 Nā nila inikini sterile hoʻolei ʻia E ʻike i ke code kala
ISO 7864:2016 Nā nila inikini sterile hoʻolei ʻia
ISO 9626:2016 Nā paipu nila kila kila no ka hana ʻana i nā mea lapaʻau

He alakaʻi ʻo SHANGHAI TEAMSTAND CORPORATION i nā huahana a me nā hoʻonā lapaʻau.
Me ka ʻoi aku o 10 mau makahiki o ka ʻike hoʻolako olakino, hāʻawi mākou i kahi koho huahana ākea, nā kumukūʻai hoʻokūkū, nā lawelawe OEM kūikawā, a me nā hoʻouna manawa kūpono. ʻO mākou ka mea hoʻolako o ke Keʻena Ola o ke Aupuni Australia (AGDH) a me ke Keʻena Ola o Kaleponi (CDPH). Ma Kina, kūlana mākou ma waena o nā mea hoʻolako kiʻekiʻe o Infusion, Injection, Vascular Access, Rehabilitation Equipment, Hemodialysis, Biopsy Needle a me Paracentesis.
Ma ka makahiki 2023, ua hoʻolako pono mākou i nā huahana i nā mea kūʻai aku ma nā ʻāina he 120+, me ʻAmelika Hui Pū ʻIa, EU, Hikina Waena, a me ʻAsia Hikina Hema. Hōʻike kā mākou hana i kēlā me kēia lā i ko mākou hoʻolaʻa a me ka pane ʻana i nā pono o nā mea kūʻai aku, e hoʻolilo ana iā mākou i hoa ʻoihana hilinaʻi a hoʻohui ʻia o ke koho.

Ua loaʻa iā mākou kahi inoa maikaʻi ma waena o kēia mau mea kūʻai aku a pau no ka lawelawe maikaʻi a me ke kumukūʻai hoʻokūkū.

A1: He 10 mau makahiki o ko mākou ʻike ma kēia kahua, he hui ʻoihana kā mākou hui a me ka laina hana ʻoihana.
A2. ʻO kā mākou huahana me ke kūlana kiʻekiʻe a me ke kumukūʻai hoʻokūkū.
ʻO A3.ʻO ka maʻamau he 10000pcs; makemake mākou e hana pū me ʻoe, mai hopohopo e pili ana i ka MOQ, e hoʻouna wale mai iā mākou i kāu mau mea āu e makemake ai e kauoha.
ʻAe, ua ʻae ʻia ka hoʻopilikino LOGO.
A5: ʻO ka maʻamau mālama mākou i ka hapa nui o nā huahana i loko o ka waihona, hiki iā mākou ke hoʻouna i nā laʻana i loko o 5-10 mau lā hana.
A6: Hoʻouna mākou ma FEDEX.UPS, DHL, EMS a i ʻole ke Kai.